
O3 Lewis StructureOzone Lewis StructureLewis Dot Structure for O3
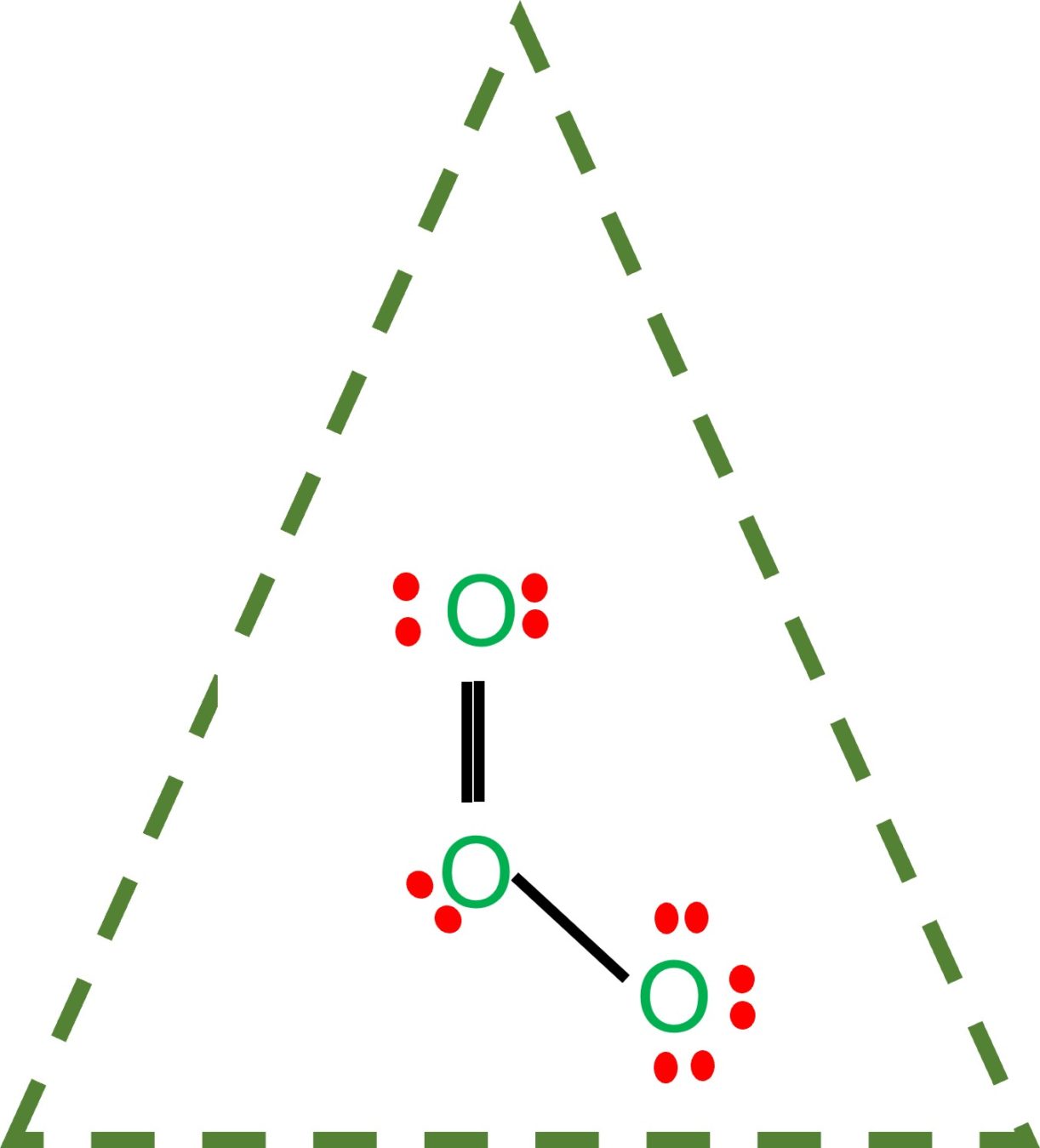
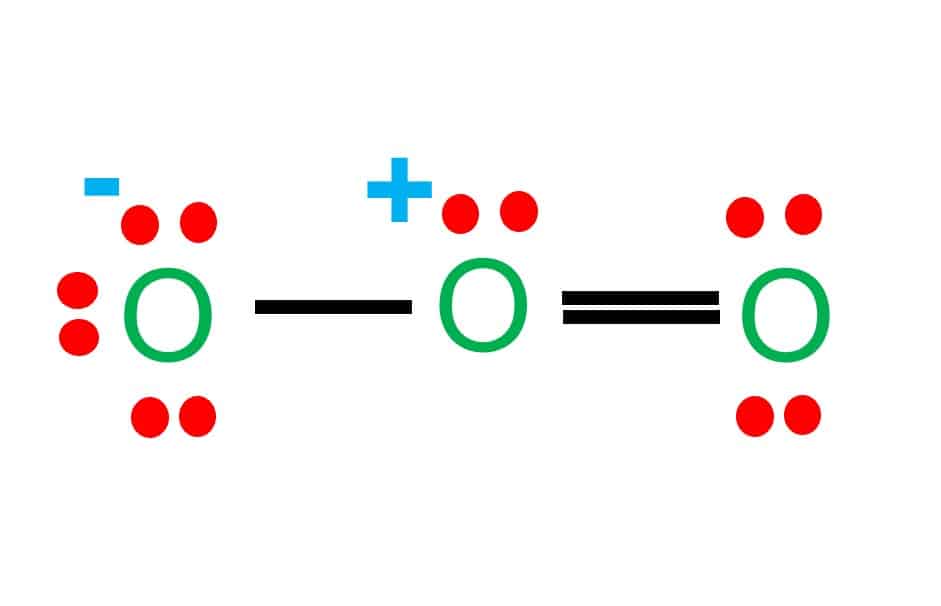
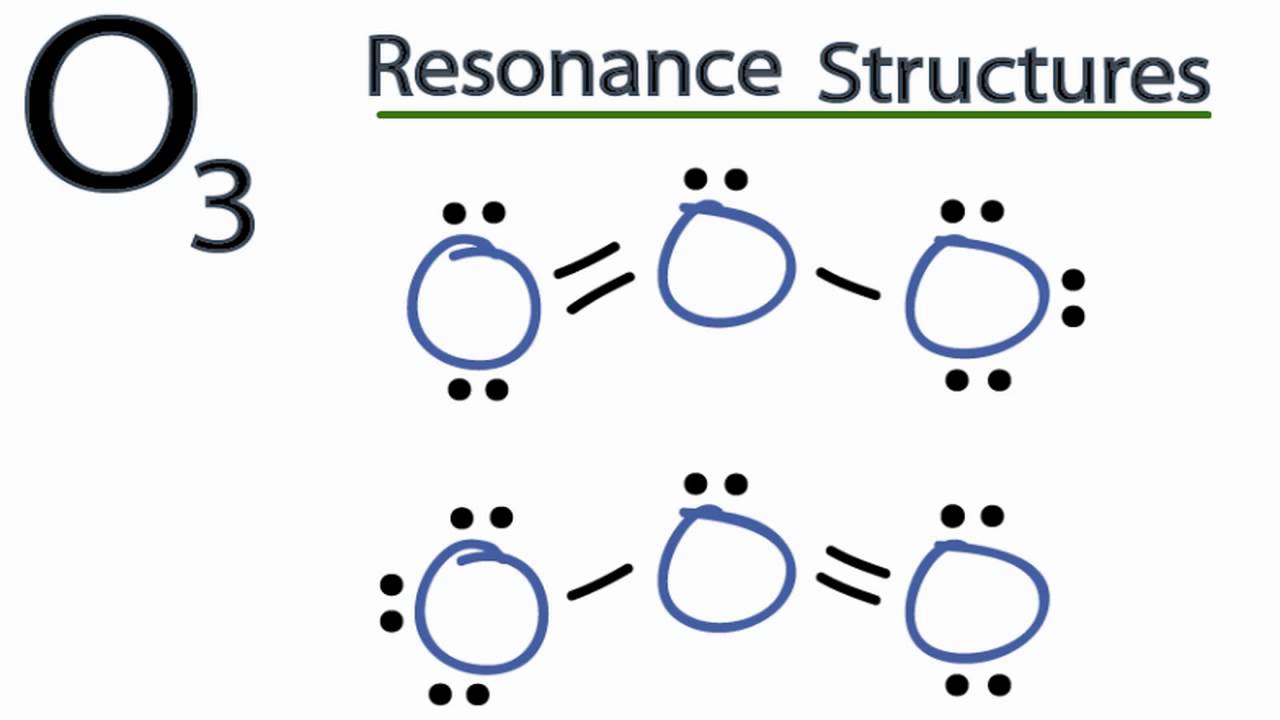
The ozone (O 3) molecule has two equivalent octet structures, shown below: In both cases, the Lewis dot diagram suggests that there are three kinds of oxygen atoms in the molecule, with +1, 0, and -1 formal charges. These structures also suggest that ozone should have one single and one double bond.
O3 Lewis Structure Step By Step Drawing What's Insight
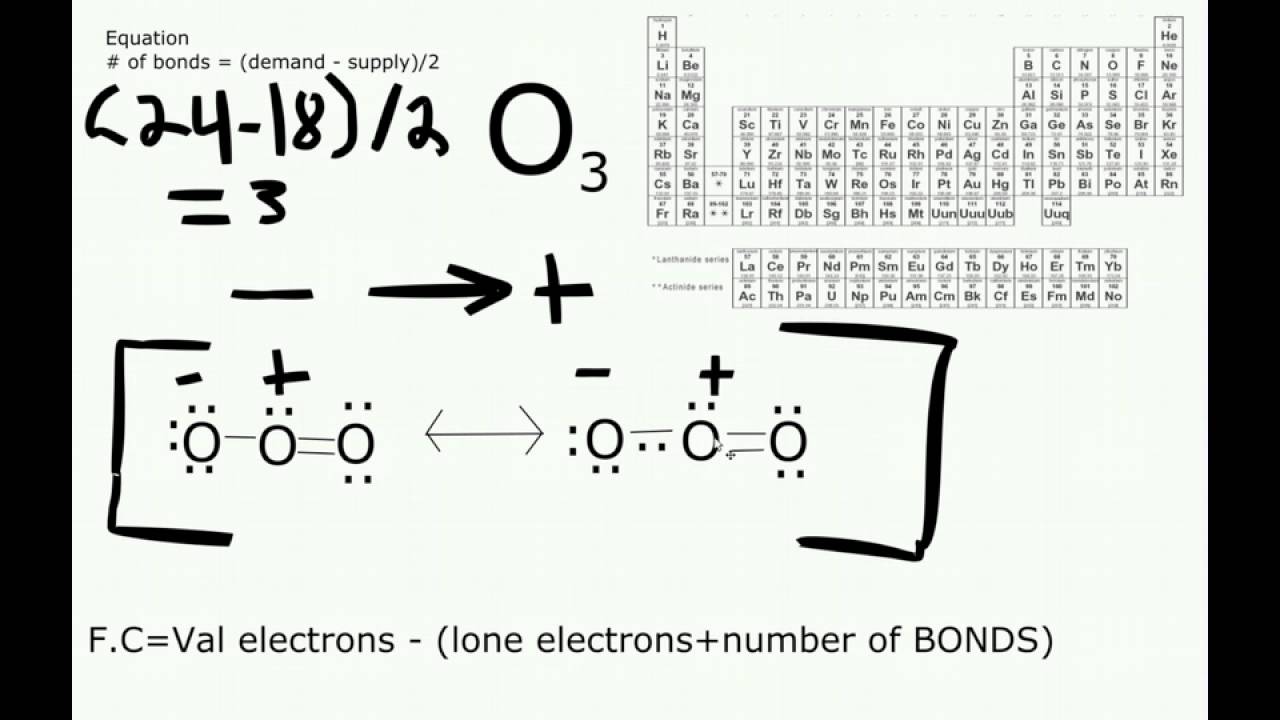
Ozone (O3) is an allotrope of oxygen and contains three oxygen atoms. In the lewis structure of ozone, there are one double bond and one single bond. Also, there are charges in two oxygen atoms in O 3 lewis structure. Lewis structure of O 3 can be drawn by starting from valence electrons of oxygen atoms in several steps.

Ozone Molecule Lewis Structure
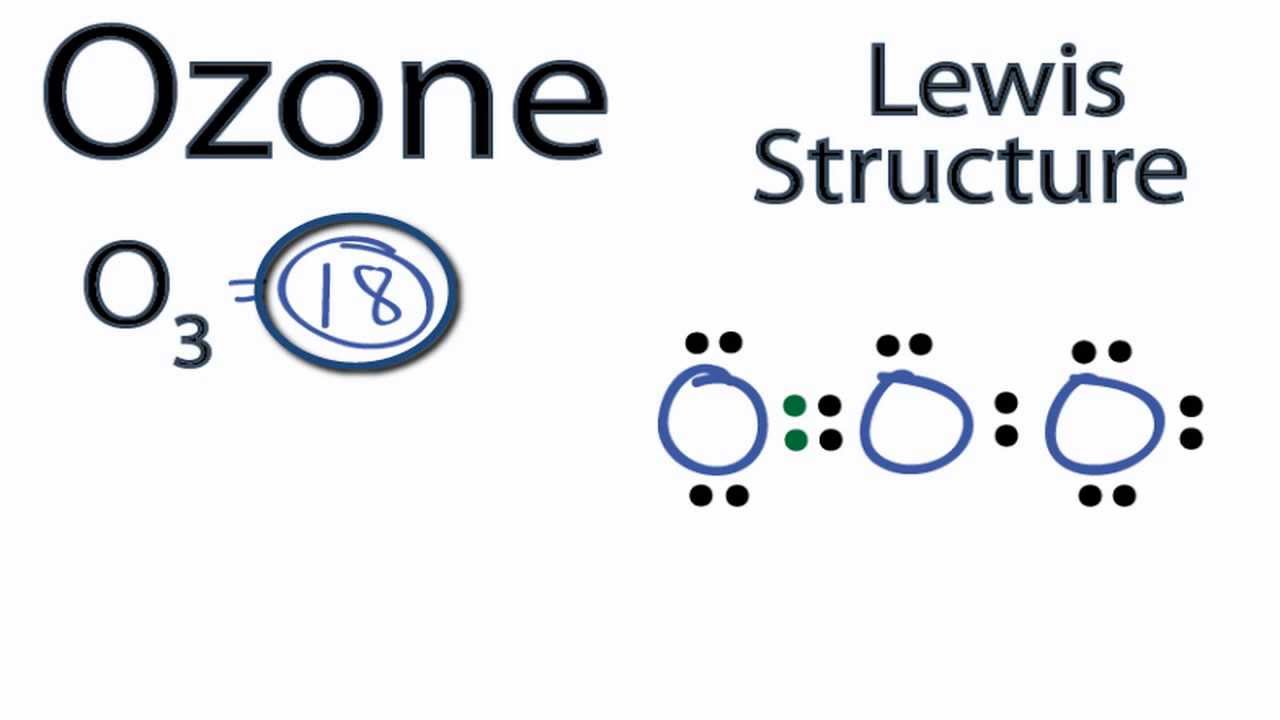
A step-by-step explanation of how to draw the O3 Lewis Dot Structure (Ozone). For the O3 structure use the periodic table to find the total number of valence electrons.more.more.
O3 Lewis Structure Ozone YouTube
Ozone, sometimes referred to as smog, is a gas formed in the atmosphere when three atoms of oxygen combine. The chemical structure of ozone is the same wherever it is found; however, there are two categories of ozone: Stratospheric and ground-level. Stratospheric ozone is found naturally in the Earth's upper atmosphere — six to 30 miles.
Ozone Lewis Structure How to Draw the Lewis Structure for Ozone YouTube
A step-by-step explanation of how to draw the O3 Lewis Dot Structure (Ozone).For the O3 structure use the periodic table to find the total number of valence.

O3 Lewis Structure (Ozone) Lewis, Ozone, Chemistry
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, and.

Oxygen Valence Electrons (O) Oxygen Valency & Electron Configuration
Lets start with a look at the Lewis Dot Structure of Ozone. Illustration of Resonance: Ozone. Ozone (O 3) is an allotrope of oxygen, with diatomic oxygen (O 2) being the most common form of oxygen. Ozone is a very reactive form of oxygen that has detrimental health effects (which is why ozone alerts are posted along the highways of cities), but.

Single Oxygen Lewis Structure
A step-by-step explanation of how to draw the O3 Lewis Dot Structure (Ozone).For the O3 structure use the periodic table to find the total number of valence.
O3 Lewis Structure Step By Step Drawing What's Insight
Ozone, sometimes referred to as smog, is a gas that is formed in the atmosphere when three atoms of oxygen combine. The chemical structure of ozone is the same wherever it is found; however, there are two categories of ozone. Stratospheric Ozone is found naturally in the Earth's upper atmosphere - 6 to 30 miles above the Earth's surface.
Ozone Molecule Lewis Structure
Equivalent Lewis dot structures, such as those of ozone, are called resonance structures . The position of the atoms is the same in the various resonance structures of a compound, but the position of the electrons is different. Double-headed arrows link the different resonance structures of a compound:

Stucture of Ozone O3 Lewis Dot method YouTube
1K 84K views 3 years ago New AP & General Chemistry Video Playlist This chemistry video tutorial explains how to draw the lewis structure of O3. It also discusses the molecular geometry, bond.

Ozone Molecule Lewis Structure
Sometimes one Lewis Structure is not Enough . Some molecules or ions cannot be adequately described by a single Lewis structure. For example, drawing one Lewis structure for ozone (O 3) gives us a misleading picture of the actual bonding in the molecule.If we draw a Lewis structure for O 3 (ozone), we get this:. This structure predicts that the two bonds are different lengths and strengths.

O3 Lewis Structure Ozone YouTube
For starters, the Lewis dot structure for ozone does not look like that. It is not a ring. This is explained in this link: Can ozone have a triangular structure? There it is also given (one of the simpler answers): In order to form a 3-member ring your electron pairs would have to bend at 60 degrees; the energy required to push naturally.

O3 Lewis Structure How to Draw the Dot Structure for O3 YouTube
Ozone (/ ˈ oʊ z oʊ n /) (or trioxygen) is an inorganic molecule with the chemical formula O 3.It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope O 2, breaking down in the lower atmosphere to O 2 ().Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the.
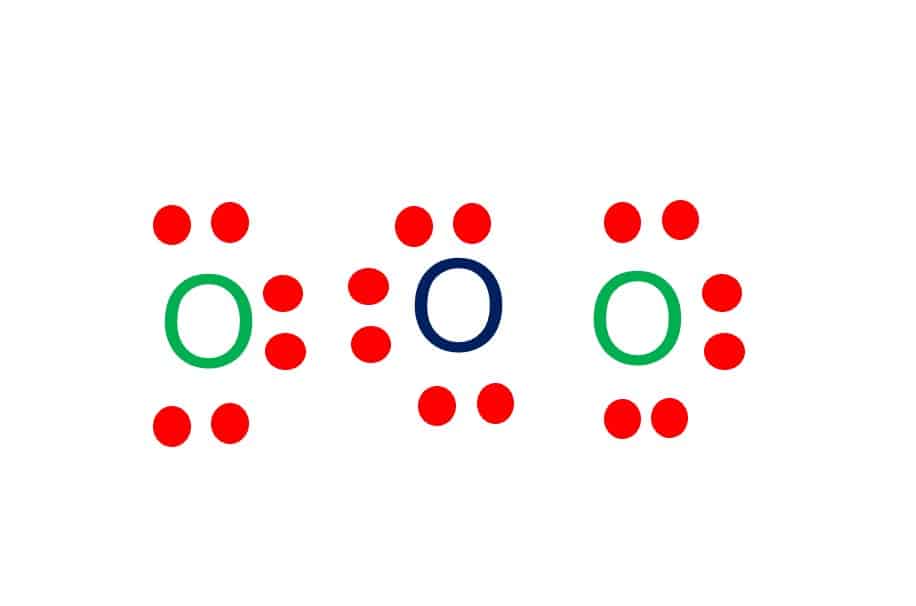
O3 Lewis Structure Step By Step Drawing What's Insight
Lewis Structure To be very precise, Lewis Structure is the name given to the structural representation of a molecule. It is the diagrammatic layout for understanding the nitty-gritty of chemical bonding. A very essential concept of molecular chemistry, the following steps dictate how you can successfully draw Lewis Structure: Step 1
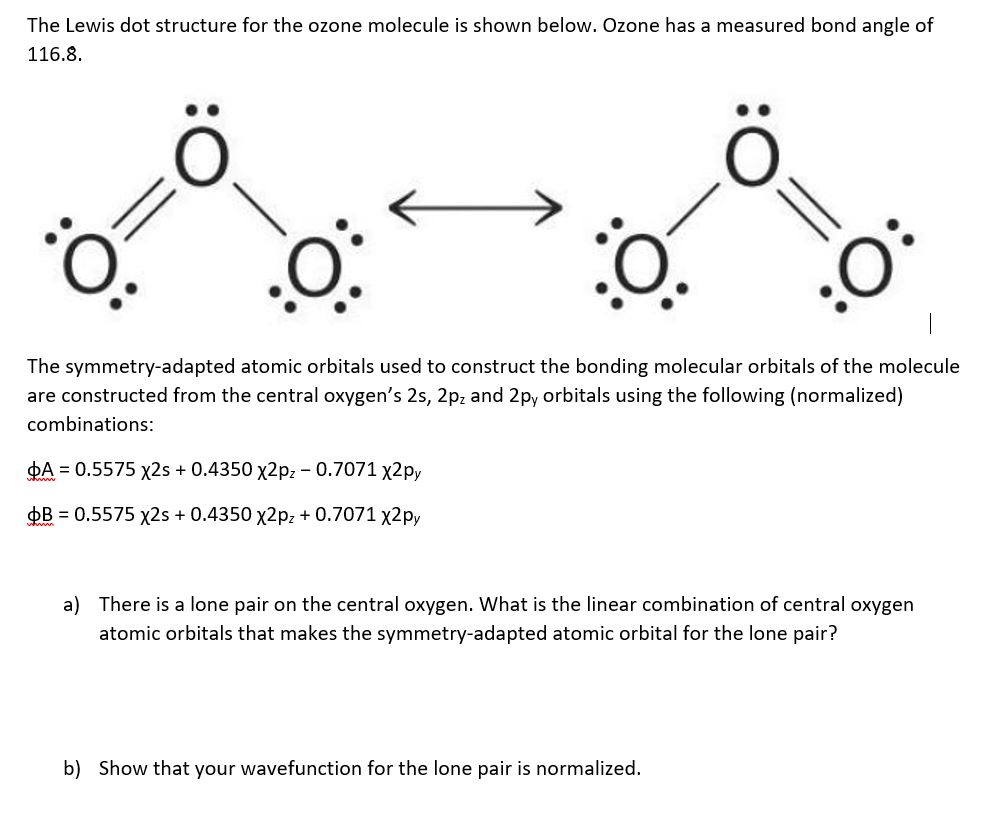
Solved Draw The Lewis Structure For The Ozone Molecule Chegg Com My
0:00 / 1:59 Lewis Dot Structure of O3 (Ozone) kentchemistry.com 24.7K subscribers 131K views 11 years ago I quickly take you through how to draw the Lewis Structure of O3 (Ozone). I also go.